
A Framework for Spreadsheet Validation
With the release of the Computer Software Assurance for Production and Quality System Software by the FDA, we get a clear framework to apply for spreadsheet validation.

With the release of the Computer Software Assurance for Production and Quality System Software by the FDA, we get a clear framework to apply for spreadsheet validation.

The life sciences industry is a complex and dynamic field, where (independent) consultants play a critical role in providing specialized expertise to companies. However, navigating relationships with business managers and headhunters can be challenging, especially when their actions or decisions negatively impact the consultant’s experience and effectiveness. In this article, we will explore some of…

In Good Manufacturing Practices (GMP) environment, ensuring that systems and equipment meet regulatory standards is critical. Two pivotal documents that play significant roles in this process are the Design Specification (DS) and the User Requirements Specification (URS). While both are essential, they serve distinct purposes and are developed at different stages of a project. What…

In the dynamic field of Good Manufacturing Practices (GMP), ensuring the integrity, efficiency, and compliance of equipment is a requirement. This article looks into the essential aspects of periodical equipment reviews within GMP environments, highlighting their significance, processes, and outcomes. The Necessity of Periodical Equipment Reviews Regular reviews are crucial for maintaining equipment functionality and…

In the pharmaceutical industry, Computer System Validation (CSV) is a critical process that ensures the accuracy and reliability of computer systems used in manufacturing and quality control. This article explores the differences between CSV processes for production equipment and QC (Quality Control) equipment, highlighting their unique requirements and significance in ensuring product quality and regulatory…

In the dynamic and regulated world of pharmaceutical manufacturing, ensuring compliance with Good Manufacturing Practices (GMP) is non-negotiable. At the heart of these practices lies the importance of Computer System Validation (CSV), which ensures that all computer systems used in manufacturing and quality control are reliable, accurate, and consistent. Within this framework, User Requirements Specifications…

Regulatory Requirements for the Validation of Legacy Systems Legacy systems pose unique challenges due to their age and potential obsolescence. Regulatory bodies like the FDA have specific guidelines that apply to the validation of legacy systems, but these guidelines mostly apply in the context of systems that were operational prior to August 20, 1997, the…

Introduction Good Manufacturing Practices (GMP) are the cornerstone of the pharmaceutical industry, ensuring that pharmaceutical products are produced consistently, safely, and effectively. The validation process is a critical component of GMP compliance, as it verifies that equipment and processes meet predefined standards and specifications. However, deviations and incidents during this validation process can have both…

In the pharmaceutical industry, Good Manufacturing Practices (GMP) are fundamental to ensuring that products meet the highest standards of quality, safety, and efficacy. A critical component of GMP compliance is validation, which proves that equipment and processes consistently produce results as intended. Traditionally, validation is a resource-intensive process, often applying uniform rigor across all equipment…
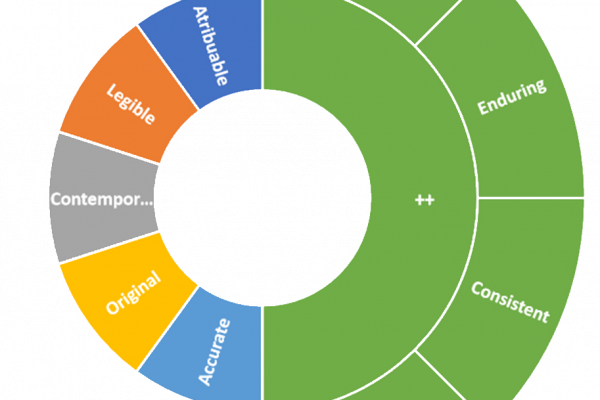
In the pharmaceutical industry, data integrity plays a pivotal role in maintaining the quality, safety, andefficacy of their products. Good Manufacturing Practices (GMP) are designed to ensure that all aspects of drug production adhere to regulatory standards, with data integrity being a cornerstone of these practices. This article delves into the importance of data integrity…